The scientific community widely discusses preregistration. The main idea of preregistration is being able to untangle the a priori hypothesis from the outcome-driven and exploratory analyses once the data is generated. Researchers can ensure neutrality towards their data and an objective evaluation of the study outcomes by cultivating a record of the study, starting with the study plan. This record is especially valuable during the peer review process by assisting publishers in retracing the hypothesis generation and data analysis.
Researchers have a couple of options for sharing their methods, plans, statistical analyses, and results with publishers: as a study protocol in a public registry (database) or as a registered report in a journal. The content of a preregistered study depends on the requirements of the database or journal. It can range from detailed study and analysis protocols to a simplified documentation of the exploratory process of data collection without an explicit plan for data evaluation. Publishers’ confidence in the reproducibility of research findings grows the more comprehensively the experimental plan is documented a priori, including what the authors expect to find and what these findings will mean. Public registries can offer multiple advantages and, if supported by journals, open up preregistration to a broader range of researchers. The requirement by the International Committee of Medical Journal Editors that clinical trials need to be registered prior to publication was an important milestone for the acceptance of preregistration among clinical researchers. The adoption of similar strategies by journals in preclinical and fundamental research could result in an improvement of study quality and reproducibility in these fields.
The Value of Public Registries
A recent Science Editor article1 illustrated the key features of registered reports. In brief, researchers submit the introduction, methods description, and analysis plan of their study for peer review in a journal prior to performing the experiments. Once the proposal is accepted, it is registered and, assuming that the authors have followed the submitted protocols, the publication of the results does not depend on the study outcomes. This concept directly affects publication bias—a strong preference for publication of positive findings. Furthermore, it promotes the idea that well thought-out and planned experiments are significant regardless of their outcomes.
In this commentary, we highlight preregistration of study protocols in public registries. It requires researchers to write a detailed protocol of the study plan, including a description of the methods and statistical planning, which is then time stamped and saved in a permanent database. Similar to registered reports, they have the potential for accurate documentation of study designs, and their record can be submitted to a journal together with the manuscript. The key difference lies in the faster registration due to the absence of a peer-review process, which can include several steps with revisions. Preregistration could offer an easier solution if, for example, at the beginning of the project, the peer-review process as carried out by peers from the same research area is met with reservations. Because of embargo periods offered by the preregistration platforms, the study plan would not necessarily be made public at the beginning of the study.
In most registries (e.g., preclinicaltrials.eu2 or Open Science Framework Preregistration3), other researchers can still access registered study designs and compare them to the published data after the embargo period is over (for an overview of preregistration platforms for preclinical studies, see Table 1). However, other registries such as the As Predicted platform (launched by the Wharton Credibility Lab of the Wharton School of the University of Pennsylvania in 2015)4 allow the completed records to be private for an unlimited time. Once the study is ready to publish, the record of preregistration can serve as a foundation for the manuscript, saving additional time and resources at the submission stage. Reviewers and editors can verify that the study was conducted according to the study plan by comparing a preregistered study together with the submitted manuscript. The initial documentation of the study proposal together with the time stamp may provide further assurance for researchers and publishers if concerns about the research’s originality arise.
Table 1. Preregistration platforms for preclinical studies.
| Platform | Host | Launch | Scientific Focus |
|
Animal Study Registry |
German Federal Institute for Risk Assessment, Germany | 2019 | Animal studies |
|
As Predicted |
Wharton Credibility Lab, Wharton School of the University of Pennsylvania, USA | 2015 | All studies |
|
OSF Preregistration |
Open Science Framework, USA | 2013 | All studies |
|
Preclinicaltrials.edu |
Utrecht University, The Netherlands | 2018 | Animal studies |
Using open registries allows for more flexibility in terms of adaptation of the initial plan and the choice of a journal for submission. Frequently, it will make sense to test additional hypotheses and to include alternative data analyses with those anticipated in the preregistration. Exploration has always been an important part of the scientific process. However, having the option of preregistration and reporting these analyses as expansions of the planned protocol adds to the overall transparency and prevents often unintentional and questionable research practices such as p-hacking and HARKing (Hypothesizing After Results are Known).5 Further, follow-up studies or small parts of a larger research project can be easily preregistered using already existing study templates. The choice of a journal to which a manuscript is submitted is left to the researcher and often is made according to the study results. Preregistration fosters transparency on the researcher’s side, but there is no obligation by the journal to publish the outcomes of a preregistered study. In order to be able to connect a preregistered study to the published data and to address publication bias, it is crucial that preregistration platforms provide the possibility to link a study to the respective publication(s) or data repositories.
The Animal Study Registry
Preregistration in a public registry offers numerous advantages to researchers, for example, in assisting with the statistical analysis and the overall study planning process.6 However, recent evaluations estimate that the proportion of preregistered studies will not increase by itself and depends on external incentives.7 For human clinical trials, preregistration of medical interventions and treatment research is now required by law as well as medical journals.8 This policy makes all findings available to the decision makers, health professionals, and patients who weigh in on the decision whether to implement a treatment. One of the well-established clinical trial registries, clinicaltrials.gov,9 has registered over 350,000 research studies from 216 countries. Another platform open for registrations from different research areas and run by the Open Science Framework has records of around 319,000 entries, most of which are for studies from psychology and the social sciences.3 For other types of studies, its endorsement by publishers and editors can encourage the use of preregistration.10 In particular, the poor reproducibility and transferability of results from animal studies into clinical research in humans has influenced the credibility of the entire field of animal research.11 Here, a poor experimental design and an incomprehensible execution or analysis of planned animal experiments not only have significance for external validity but also for animal welfare and the guiding principles of the 3Rs by William Russel and Rex Burch (Replacement, Reduction and Refinement12). Superfluous animal experiments or their “unethical” use is often the consequence.6 By following a series of animal studies from their approval by an animal ethics committee to publication, a recent study found out that in the sum of all publications only 26 % of the used animals were reported.13
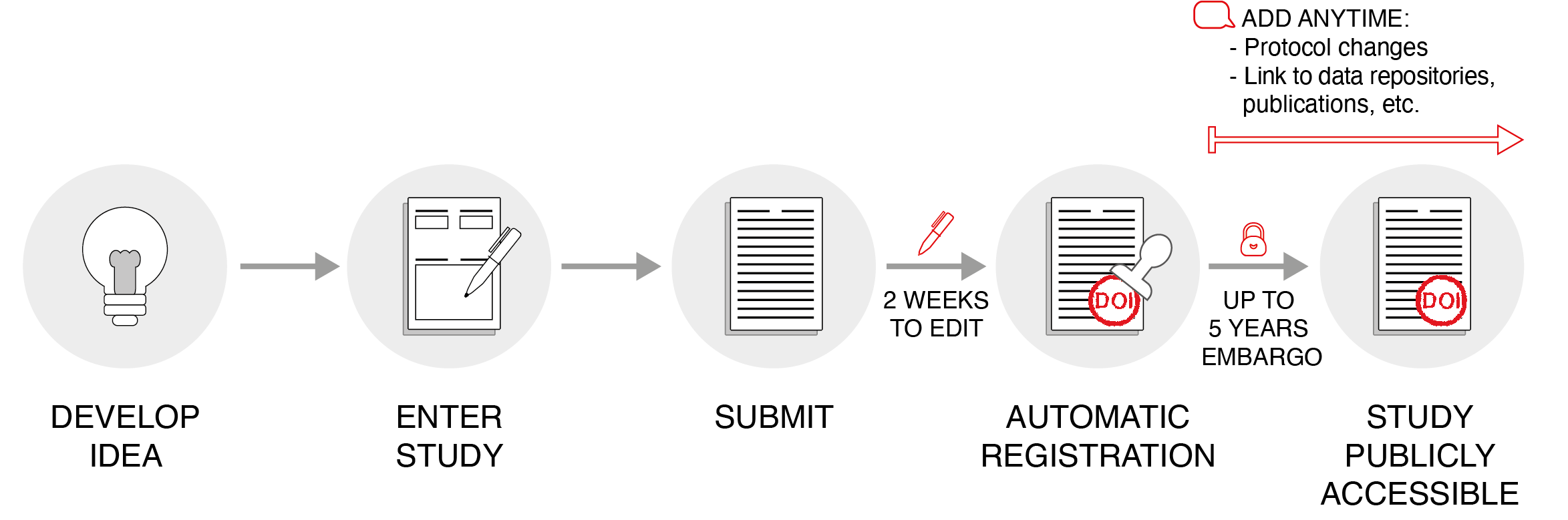
The public and free platform Animal Study Registry (ASR)14 has been developed for registering exploratory and confirmatory animal experiments in applied and preclinical science with a focus on animal welfare.15 Similar to other registries, it guides researchers through planning, execution, documentation, and statistical analysis of their studies (Figure 1).16,17 However, during the registration process, scientists have to answer animal experiment–specific questions. The questions are based on the ARRIVE Guidelines for reporting animal research and are essential for reproducibility and the peer-review process for animal research.18 Once submitted, the authors can edit a study for next 2 weeks before it is automatically registered. In parallel, the ASR confirms that the submitted study meets basic requirements: 1) the study is written in English, 2) animals are involved, and 3) the content is nonoffensive. A study automatically receives a digital object identifier (DOI) following registration, which supports the manuscript peer-review process and marks the intellectual property of the author. Thereafter, an embargo period of up to 5 years can be applied to every registered study before it becomes publicly available. After registration, a printed version of the registered study, including full study details and metadata, can be downloaded and submitted together with the manuscript to publishers irrespective of the embargo. Study authors can add comments at any time after study registration. This allows researchers to explain changes to the original study and, most importantly, provide links to data repositories or publications.
Implementation in the Publication Process
There is consensus about the potential of preregistration for reducing the irreproducibility of research data. However, it can be a challenge for researchers to go against the accepted practice of not sharing their work until they write the manuscript. For the preregistration platforms to contribute more effectively to improving the transparency and quality of animal research, journals and publishers now have the task of responding to the increased need for preregistration of research projects. The earlier the communication, the more researchers can be reached at the initial stages of their projects.
Comparing public registries to registered reports offers the opportunity for journals to benefit from the advantages of preregistration without additional costs (Table 2). Some journals have already recognized the added value of preregistration to the submission and reviewing processes and are taking steps towards this. One example is the endorsement of animal experiments preregistration by the American Association of Cancer Research in their editorial policies.10 However, preregistration needs more active advocacy.
Table 2. Integration of preregistration in the publication process.
| Submission |
|
| Manuscript peer review |
|
Asking authors additional questions during the manuscript submission process about whether the study has been preregistered can help to disseminate preregistration in the research community and might encourage scientists to register their follow-up studies. Journals could choose to clearly label preregistered studies and thus acknowledge the efforts of the authors who have already endorsed preregistration. Likewise, editors can raise awareness on the reviewer’s side: Because reviewers might not yet be familiar with the concept of preregistration, advertising public registries may not only help with reviewing the received manuscripts, but also motivate them as scientists to register their own studies. Our concern is that without clear recommendations, guidelines, or policy from publishers—similar to the adoption of preregistration in clinical research—we leave researchers in an ambiguous position. To prevent selective reporting and unnecessary duplication, and to increase the reproducibility of preclinical and fundamental research, the endorsement of preregistration as pioneering work by journals is pivotal. This is especially the case for the preregistration of animal studies.
References and Links
- Kousta S, Pastrana E, Swaminathan S. Three approaches to support reproducible research. Sci Ed. 2019;42(3):77–82. [accessed February 8, 2021]. https://www.csescienceeditor.org/article/three-approaches-to-support-reproducible-research/.
- https://preclinicaltrials.eu/
- https://osf.io/prereg/
- https://aspredicted.org/
- Dirnagl U. Preregistration of exploratory research: learning from the golden age of discovery. PLoS Biol. 2020;18(3):e3000690. https://doi.org/10.1371/journal.pbio.3000690
- Heinl C, Chmielewska J, Olevska A, Grune B, Schönfelder G, Bert B. Rethinking the incentive system in science: animal study registries: preregistering experiments using animals could greatly improve transparency and reliability of biomedical studies and improve animal welfare. EMBO Rep. 2020;21(1):e49709. https://doi.org/10.15252/embr.201949709
- Sherry CE, Pollard JZ, Tritz D, Carr BK, Pierce A, Vassar M. Assessment of transparent and reproducible research practices in the psychiatry literature. Gen Psychiatr. 2020;33(1):e100149. https://doi.org/10.1136/gpsych-2019-100149
- European Commission. Clinical trials – regulation EU No. 536/2014; revision of section 6.
- https://clinicaltrials.gov/
- American Association for Cancer Research. Editorial policies. [accessed February 8, 2021]. https://aacrjournals.org/content/authors/editorial-policies.
- Bailoo JD, Reichlin TS, Würbel H. Refinement of experimental design and conduct in laboratory animal research. ILAR J. 2014;55(3):383–391. https://doi.org/10.1093/ilar/ilu037
- Russell WMS, Burch RL. The principles of humane experimental technique. London, UK: Methuen; 1959.
- van der Naald M, Wenker S, Doevendans PA, Wever KE, Chamuleau SAJ. Publication rate in preclinical research: a plea for preregistration. BMJ Open Sci. 2020;4:e100051. https://doi.org/10.1136/bmjos-2019-100051
- animalstudyregistry.org
- Bert B, Heinl C, Chmielewska J, Schwarz F, Grune B, Hensel A, Greiner M, Schönfelder G. Refining animal research: The Animal Study Registry. PLoS Biol. 2019;17(10):e3000463. https://doi.org/10.1371/journal.pbio.3000463
- Nosek BA, Ebersole CR, DeHaven AC, Mellor DT. The preregistration revolution. Proc Natl Acad Sci U S A. 2018;115(11):2600–2606. https://doi.org/10.1073/pnas.1708274114
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DChamuleau SAJ, van der Naald M, Climent AM, Kraaijeveld AO, Wever KE, Duncker DJ, Fernández-Avilés F, Bolli R; Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes (TACTICS) Group. Translational research in cardiovascular repair: a call for a paradigm shift. Circ Res. 2018;122(2):310–318. https://doi.org/10.1161/CIRCRESAHA.117.311565
- Improving Bioscience Research Reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology 2010;8(6):e1000412. https://doi.org/10.1371/journal.pbio.1000412
Anastasia Olevska (0000-0003-0612-1631), Bettina Bert (0000-0002-8202-9290), Lida Ebrahimi, and Céline Heinl (0000-0003-3369-1939) are with German Federal Institute for Risk Assessment, German Centre for the Protection of Laboratory Animals (Bf3R), Berlin, Germany. Gilbert Schönfelder (0000-0001-6134-1990) is with German Federal Institute for Risk Assessment, German Centre for the Protection of Laboratory Animals (Bf3R) and Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
