Abstract
The Editorial Services Team at the United States Pharmacopeia (USP) supports the mission of USP by partnering with in-house scientific liaisons (SLs) to publish the highest quality documentary standards. We provide developmental reviews, copyediting and proofreading, quality checks, print and online production reviews, and quality and workload tracking for the general chapters, monographs, and reagents comprise USP our publications. Editorial Services management conducted a longitudinal study with the hypothesis that if substantive editorial reviews are performed earlier in the process, this would result in higher quality, less workload variability, and a reduction of deferrals and errata. This study evaluated the optimization of the team along a task-based staffing model, replacing the previous publication-based model, and identified methods to continuously improve author support. A series of qualitative surveys were conducted to aggregate staff skills and engagement with specific segments of the editorial process. We reviewed the results, then piloted and implemented several new processes. In addition, we removed duplicate steps in the workflow and created more focused workflow stages, such as developmental review and quality control. The results of this study are impactful for both internal and external stakeholders. The workflow is more streamlined and accurate, with more robust, specific work instructions and checklists to ensure that we consistently publish high quality content. This new workflow strengthens ties with authors by providing more support during early monograph development and streamlines file handoff. It also allows for more focused scientific peer review, removing the correction of minor grammatical errors and structural questions from the reviewer’s responsibilities.
Introduction
Our team edits and reviews content that publishes in the USP-NF, Pharmacopeial Forum (PF); Food Chemical Codex (FCC), Dietary Supplements Compendium (DSC); as well as monthly Accelerated Revisions and Errata; and several other publications for USP. This content primarily consists of documentary standards, which contain the necessary tests, procedures, acceptance criteria, and other requirements necessary for developing the drug and for storing it. The documentary standards help to assess the quality, strength, identity, and purity of chemical medicines, biologics, excipients, food chemicals and ingredients, dietary supplements, and other items. Our content is unique compared with other scientific publications, because many of USP’s published standards are enforceable by federal law under the Federal Food, Drug and Cosmetic Act of 1938.
In order to effectively manage the increasing workload of submissions by the authors in our Science Division, we had to think of new and inventive ways to improve the way we work. We conducted significant background research, piloted several new processes over a 2.5-year period, and ultimately implemented a new team structure, improved editorial workflow stages, and a better mechanism for measuring quality and the ultimate impact of our publications. Our previous editorial workflow and team structure was based on processing our 2 largest publications, PF and USP–NF. Editorial staff worked primarily on their assigned publication with little overlap in resources, and the workflow had redundancies and inaccurate task identifiers.
Methods
Background Research
Staff surveys. In December 2018, scientific editors were asked to complete a survey and rate the various tasks performed in their roles. This information helped guide editorial leadership in their decisions about how to structure the Editorial Services Team to better align staff expertise and skills with job function.
Task-based staffing model. In February 2019, the Editorial Services Team met to discuss the survey results and align on a proposed “future state” for the team structure. Team leads and managers led the discussion and outlined a detailed staffing model to be implemented. This staffing model was piloted over several months and eventually implemented at the beginning of fiscal year 2020.
Publishing industry best practices. We researched publishing industry best practices and discovered that earlier editorial participation has a positive impact on the overall quality of a publication. This approach was later supported by the recommendations of KWF Consulting, who independently evaluated the USP publication workflow.1 We modeled our task definitions on standard workflows for scientific and technical publications that we felt met the requirements of USP’s specific needs.2–4
Pilot Implementation
The original pilot began in July 2017 when the Editorial Services Team conducted “functional reviews” of new and revised general chapters proposed for PF. This effort was instituted to improve the quality of content prior to submission, reducing rework performed by both the production and editorial teams. Starting in July 2018, this review expanded to a selection of complex monographs and was renamed “pre-submission review.” Scientific editors were asked to track turnaround time factors such as meetings, rounds of queries, and time spent incorporating changes.
After continued research into publishing industry best practices, incorporating feedback from stakeholders, and lessons learned from turnaround tracking, we officially kicked off the “developmental review” pilot in January 2019. This new pilot was shared with Science Divison stakeholders and leadership to ensure the smooth transition of work from each department. We provided in-depth training and coaching to our authors (SLs) to ensure they understood the benefits of this new workflow step. Editorial staff were cross-trained, beginning in March 2019, and the pilot was conducted over a 10-month period.
We hypothesized that this new step would satisfy the need for more editorial oversight early in the documentary standards development process and would optimize the team along a task-based staffing model.
Transparency and Process Updates
To support the implementation of our new process and teams, and to provide greater transparency during proposal hand off, it was necessary to clarify the tasks and steps being performed. In addition to implementing a developmental review, we removed duplicate steps in the workflow and added and/or renamed the current workflow stages as follows:
- Editorial Staging (new)
- Dev Review (formerly 1st Read, available at 4 pre-submission steps)
- Copyedit (formerly 2nd read)
- Proofread (formerly Editorial Review)
- QC (added to more stages of the workflows)
We created a QC team responsible for upholding the quality of all content by conducting critical QC reviews on all products to ensure that publication content is consistent and accurate.
Results
Editorial Turnaround
A major goal of this study was to improve workflow efficiency without compromising publication quality (see Discussion for more). Therefore, while each task was more clearly defined and redundancies removed, each workflow still required a minimum level of review and QC to ensure adherence to quality standards and SOPs. These steps include Submission Review, Dev Review, Copyedit, and QC for most file types. From this controlled state, we analyzed the variables of team structure (by task) and number of days to complete each task before and after the workflow transition.
In the previous publication-centered structure, the average turnaround time from “Submission Review” to “SL Review” was approximately 28 days. Since transitioning to task-based teams and modifying the editorial workflow to allow for Dev Review before submission to the publications department, this timeframe has decreased to approximately 18 days.
Figure 1 shows the turnaround time improvement for the QC step. In the previous workflow, scientific editors were performing all editorial tasks, with assignments centered on specific products. Because of this, PF QCs took nearly 3 days to be completed. The redistribution of tasks to the newly created Editorial Development and Quality Control teams allows work to move seamlessly through the editorial process and has improved turnaround times for PF by 1 day.
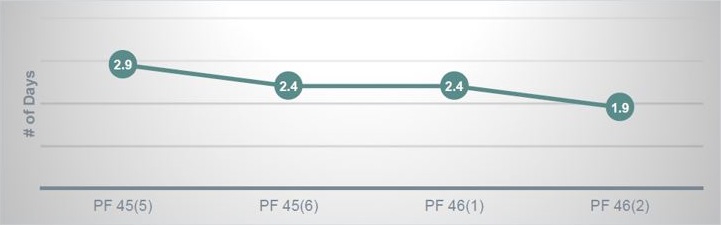
SL Galley Review
Under the previous workflow, which required all editorial reviews to take place after submission to the publications department, a segment of the galleys for each issue of PF were at risk of receiving less than a 5-day review from SLs at the end of the process. This was a result of variable submission numbers. In the new workflow, substantive work can be completed before submission, which greatly improves the speed at which galleys are delivered for final review. Figure 2 shows 2 issues of PF under the former workflow (orange), and 2 issues under the new workflow (yellow).
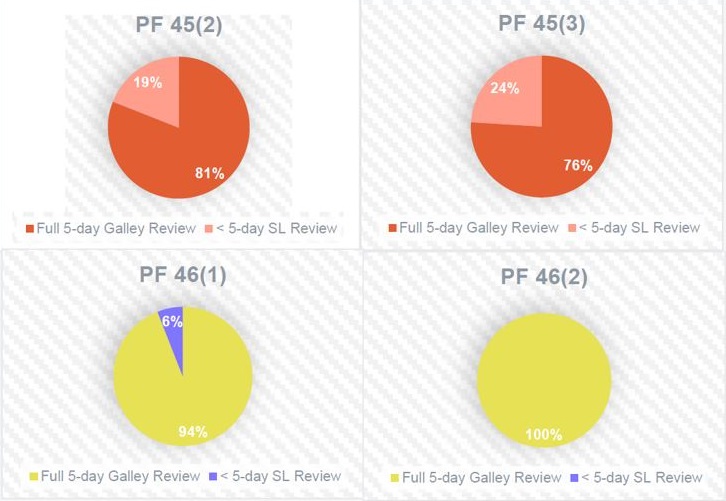
Employee Satisfaction
Throughout the restructuring process, editorial leadership ensured that individual contributors were included in the decision-making process. We conducted initial surveys to collect information about how to improve editorial processes. After transitioning to the new task-based team structure, we conducted a survey to assess the level of satisfaction of scientific editors in their new roles. The scientific editors rated their level of satisfaction at a 4.4 (5 = very satisfied).
Discussion
Analysis of Editorial Process
After aggregating staff skills, we dissected specific segments of the editorial process to determine areas for efficiency and improvement. We analyzed publication quality, workload, and turnaround times, and discovered significant workload variability across volumes of PF. This workload variability significantly impacts quality due to resource constraints during peak periods. Based on staff feedback and observed trends, we determined that reducing task variability could improve efficiency and staff satisfaction.
Specialized, high-impact tasks like Dev Review and QC became the cornerstones for each team, with lower-impact tasks like Copyedit and SL Corrections available for either team depending on workload. The distribution of high-impact tasks ensures that staff maintain an equitable and tailored portfolio of work while meeting overlapping production deadlines.
Quality
A key control factor during these experiments was editorial quality, which has been internally measured against a documented set of standards for several years. Since implementing the new workflow, editorial quality for PF is starting to show a decreasing trend in the number of critical and major errors corrected at QC, along with a reduction in the number of files that require QC corrections.
The task-based workflow now allows us to measure impact on quality at each stage in the editorial workflow, due to task-specific checklists and clear lanes of responsibility. By categorizing and aggregating the number of corrections made to PF submissions during the Dev Review, we hope to provide feedback and insights to our Science Division colleagues, with the goal of further aligning expectations and refining hand-off processes. The measurement model is adapted from similar studies in the literature.2
Figure 3 shows a preliminary heat map of developmental edits made for PF volume 45, issue5, categorized by type of catch and classified according to historical editorial quality standards. For example, most “critical” edits were made during the “Verification” portion of the review, and the highest frequency corrections were “Revision Tagging,” “Content-Missing,” and “Content-Wrong.”
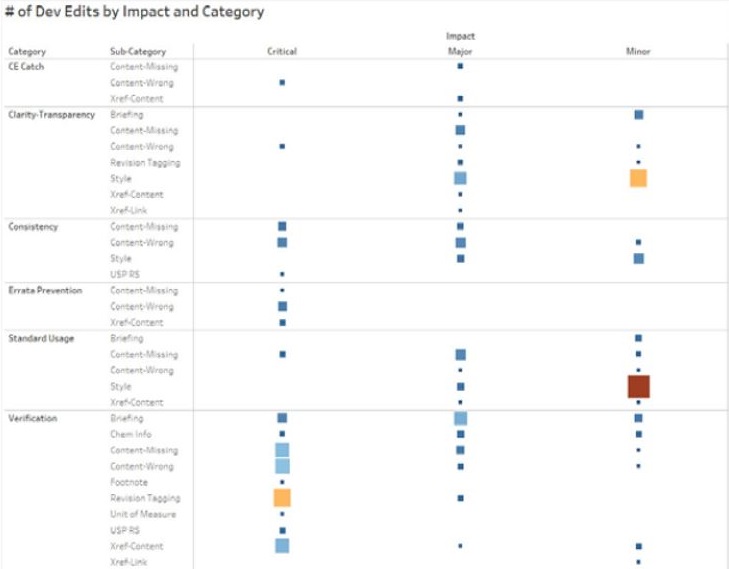
Conclusions
Support for Science
This new workflow strengthens ties with our Science Division counterparts by providing more support during early standards development, and streamlines the hand off of work. Improved turnaround times and close collaboration with Science stakeholders lead to the implementation of several options for earlier editorial support. Additionally, the development of new quality feedback mechanisms may become beneficial for optimizing technical reviews of PF proposals, allowing scientists to focus on science.
Workload Balance
These data-driven optimizations allow the Editorial Services Team to effectively balance the increasing workload of content submitted to the publications department, while also giving the SLs sufficient editorial support throughout the standards development process. We completed editorial reviews for several publications ahead of schedule, at higher quality, and turnaround times for QC have improved. These improvements can be attributed to several factors: 1) Staff skills and strengths are now aligned with specific tasks in the editorial process. 2) The previously existing workflow was made more efficient by removing duplicate steps and bolstering established checks at points in the process. 3) The more balanced workload allows each editor to focus on their specific task and enhance their editorial expertise.
Future Planning
In the coming fiscal years, we plan to collaborate with lab scientists and other staff to assess how these workflow improvements have impacted testing, material waste, prioritization, etc. Initial interviews around these topics indicate that the early correction of editorial errors could provide an estimated 10%–15% turnaround improvement for early procedure evaluation lab projects.
We are also working with the authors and our Portfolio Management Team to analyze the publication pipelines to more accurately predict anticipated workloads. This collaboration is essential to ensuring that adequate resources in the publications department are in place to help meet the necessary publication targets.
As we continue functioning in this new workflow we will assess and report further quality findings.
Recommendations
For other organizations anticipating altering their existing publication workflow, we recommend the following based on our experience optimizing the workflow within Editorial Services.
- Identify any existing bottlenecks in the workflow.
- Remove or simplify redundant steps based on quantifiable data.
- Include staff in conversations to gain their input and align their expertise with specific tasks.
- Pilot and implement on a smaller scale first to ensure any modifications are feasible and efficient.
- After full implementation, continually monitor the workflow to confirm the new process is working as it should and make necessary adjustments.
References and Links
- Lomangino K. Review of publishing processes. Baltimore, MD: KWF Consulting; 2019.
- Roth R. Understanding the importance of copyediting in peer-reviewed manuscripts. Sci Ed. 2019;42(2):51–54.
- Rinzler A. What should you expect from a developmental editor? [accessed December 17, 2020]. https://alanrinzler.com/2012/07/what-should-you-expect-from-a-developmental-editor/
- The Bay Area Editors’ Forum. Editorial services guide [accessed December 17, 2020]. http://www.editorsforum.org/what_do_sub_pages/definitions_develop_ed.php
Ashley Nusraty is Senior Manager, Editorial Services, Kaitlyn Watkins is Manager, Editorial Department, and Kelly Fleshman is Manager, Editorial Quality Control, at United States Pharmacopeia.
