Scientific publishing is evolving, and if the spike in interest about plain language summaries over the past several years is any sign of the appetite for adding open science principles to our publishing practices, then the future of accessible research is looking bright.
What Are Plain Language Summaries?
Plain language summaries (PLS) are typically short synopses of peer-reviewed journal publications that simplify the complex. PLS concisely summarize technical content in simple, jargon-free language for ease of reading and improved understanding. These summaries are intended for a broad and inclusive audience that encompasses anyone who may wish to engage with a piece of published research. This may include researchers from other specialties or of other native languages, science communicators, educators, policymakers, and the media, as well as the general public. This multistakeholder audience is essentially anyone seeking an accessible route to the scientific literature. In biomedical research, this list expands to include the likes of patients, patient advocates, caregivers, and healthcare professionals.
As a practice that is still growing and maturing, standardization is limited, and PLS currently come in many different formats (including multimedia) depending on individual journal requirements and author preferences.1 The location in which PLS can be found also varies, with journals hosting these within the main manuscript PDF and web page, in supplementary materials, or on third-party websites such as figshare.com. Some authors and research sponsors are opting to host PLS themselves if journals do not offer sufficient options; the publisher Future Science Group is even offering standalone PLS publication manuscripts (known as PLSPs). Increasingly, we are seeing PLS that are brief, text-based summaries embedded within the core manuscript alongside the technical abstract.2 Within the biomedical sphere, PLS that are formatted in this way, and tagged accordingly, can be indexed on PubMed to optimize discoverability.
The Argument for PLS
Information equity is enabled by openness and discoverability. As the scientific publishing community moves toward acknowledging our social responsibility and embracing open science as the norm, accessibility and transparency are proving to be core principles. Applying these principles by providing timely access to information has demonstrably saved lives during the COVID-19 pandemic.3 Furthermore, the public are interested in, and want access to, research information;4 and the opportunity to freely share in scientific advancements is, in fact, an enshrined human right.5 The research community and academia are usually well-respected institutions, but when it comes to the communication of science, public trust in scientists working for the private sector (including pharmaceutical companies) and science media is low.6 The COVID-19 pandemic has also shown that even academia may not benefit from the public trust they thought they once had. As scientific professionals, it is quite simply our ethical duty to communicate in a way that engenders trust and confidence.
With this in mind, we must put health literacy, “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions,”7 at the forefront of medical communication. As scientific professionals, it may be hard for us to grasp how prevalent low health literacy really is, but in the United States, up to 90% of adults may struggle to effectively use health information that is readily available in the community,8 and only 12% have proficient health literacy.9 In the United Kingdom, over 60% of adults are unable to understand and apply basic health information,10 and elsewhere in Europe, nearly 50% of adults have insufficient or problematic health literacy.11 The implications of this knowledge gap are significant since low health literacy is the strongest correlate of ill health.12
In the clinical setting, accessible and easily understandable information is vital for informed consent and shared decision-making. Owing to the speed at which medical knowledge evolves, keeping up to date with current thinking is a major undertaking but one necessary for making informed healthcare decisions; PLS may be especially beneficial to time-poor or nonspecialist healthcare professionals such as pharmacists, nurses, and family practitioners. This is also of particular importance for the rare disease community, in which clinicians outside of the relevant specialty may not be knowledgeable on the nuances of a specific disease area. PLS of peer-reviewed journal publications are often a gateway into the literature for these patient communities who frequently find themselves needing to become experts in their own diseases. PLS can also function as communication tools, providing appropriate language to facilitate effective dialogue and are of value to both clinicians and patients.13 In one survey-based study, PLS were found to be the third most valued source of online health-related information for patients with chronic illness and considered valuable for informing patient dialogue by 60% of clinician respondents.14 This offers an opportunity for patients to actively participate in healthcare decisions and strengthen their agency and autonomy, ultimately contributing to improved clinical outcomes.15
Improving the accessibility of our research through the inclusion of PLS is clearly a significant step towards bridging this knowledge gap and enhancing information equity by allowing different stakeholders to engage on an equal platform. In this way, the scientific community can demonstrate transparency and accountability and further build trustworthiness.16
Looking at PLS from another angle, providing accessible routes to the literature through the inclusion of PLS allows a broader audience to engage with research. This expands the readership and reach and improves the discoverability of scientific research and findings. Enabling readers to process information faster in turn speeds up dissemination and uptake of research, allowing media and communications stakeholders to engage with novel findings more readily,17 getting publications into the hands of target readers faster. PLS may also satisfy certain funding requirements or count toward patient and public involvement activities for researchers. In a recent analysis, publications that included a PLS were, in fact, downloaded more and accessed at greater rates than publications without a PLS.18 The evidence base for the value of PLS to authors and researchers is only growing, but it is already more than clear that PLS are simply the right thing to do.
Standards for PLS
Standards and codes of practice are standard for the publishing industry, and maintaining these is a critical component of quality assurance. Standards help to ensure consistency, safety, and functionality. In the pharmaceutical industry, which funds a great deal of biomedical research, they are also key for compliance and credibility. For example, following the introduction of the Consolidated Standards of Reporting Trials (CONSORT) guidelines for the reporting of randomized controlled trials in 2001, the published literature that then adhered to these standards was found to be improved in both completeness and quality.19 Knowing that a publication is compliant with relevant standards builds confidence in its credibility. In an era characterized by misinformation and miscommunication, ensuring that timely and accurate information is not only accessible but also reliable and trustworthy is more important than ever.
There are currently many cross-industry collaborations and existing initiatives working to build consensus on possible PLS standards. These efforts include ongoing, synergistic research and thought leadership from different stakeholders looking to gain insights into many different aspects of PLS. Research has so far ranged from journal policies and indexing functionality to end user perspectives and readership demands.20–23 Notable groups working in this area include the PLS Perspectives Working Group of the International Society for Medical Publication Professionals, the Patient Focused Medicines Development (PFMD) initiative, and the team at Future Science Group with their dedicated plainlanguagesummaries.com website, to name just a few.
Comprehensive guidance specifically on incorporating patient engagement practices into PLS development and cocreation is already available from PFMD,24 and a well-established toolkit including templates and checklists for infographic PLS, developed by Envision the Patient, has been in use for several years.25
In addition, several publishers have introduced policies and requirements for PLS that are included in submissions to their respective journals as part of their author guidelines to inform content development. This includes publisher-wide author guidelines from the likes of Adis (part of Springer Nature),26 Dove Press,27 Future Science Group28 and Taylor & Francis.29 There are also many other journal-level author guidelines that have been introduced by other publishers for use on an individual journal basis.
In September 2021, Open Pharma launched our recommendations for PLS.30 These recommendations advocate a minimum standard for PLS of peer-reviewed journal publications that are of high value, achievable, and cost and resource efficient for both journals and authors. In short, PLS for a broad, nonspecialist audience, in the style of an abstract, understandable and readable, free of technical jargon, unbiased, nonpromotional, peer reviewed, and easily accessed. With these baseline requirements met, we then very much encourage the inclusion of additional multimedia enhancements such as infographic or video PLS, or those intended for a more specific target audience (e.g., patients). Building upon standard PLS in this way can help further expand the reach and accessibility of a publication.
Lastly, the fourth iteration of the Good Publication Practice (GPP4) is expected to be published later in 2022 and, given the popularity of the topic, is likely to provide further guidance and outline best practices on publication-associated PLS.
With so many considerable, complementing efforts underway, it is clear that there is unlikely to be a one-size-fits-all solution for all publications. Rather, what is emerging is a portfolio of standards and guidelines for different types of PLS and plain language enhancements that support variety while also standardizing the practice; clear directions for the appropriateness and utility of different guidelines for different situations will be needed.
The Open Pharma Recommendations for PLS
Open Pharma is a multisponsor collaboration of pharmaceutical companies, nonpharmaceutical funders, publishers, patients, academics, regulators, editors, and societies seeking to identify and drive positive change in the publishing of pharmaceutical company-funded research. Our recommendations for PLS were initially developed by the Open Pharma Accessibility Workstream and were extensively reviewed and refined during an expert roundtable and a focused, public consultation throughout the first half of 2021. Our recommendations outline what we believe to be the minimum standard, providing concise guidance on PLS for authors, editors, and other stakeholders involved in PLS development (Figure).
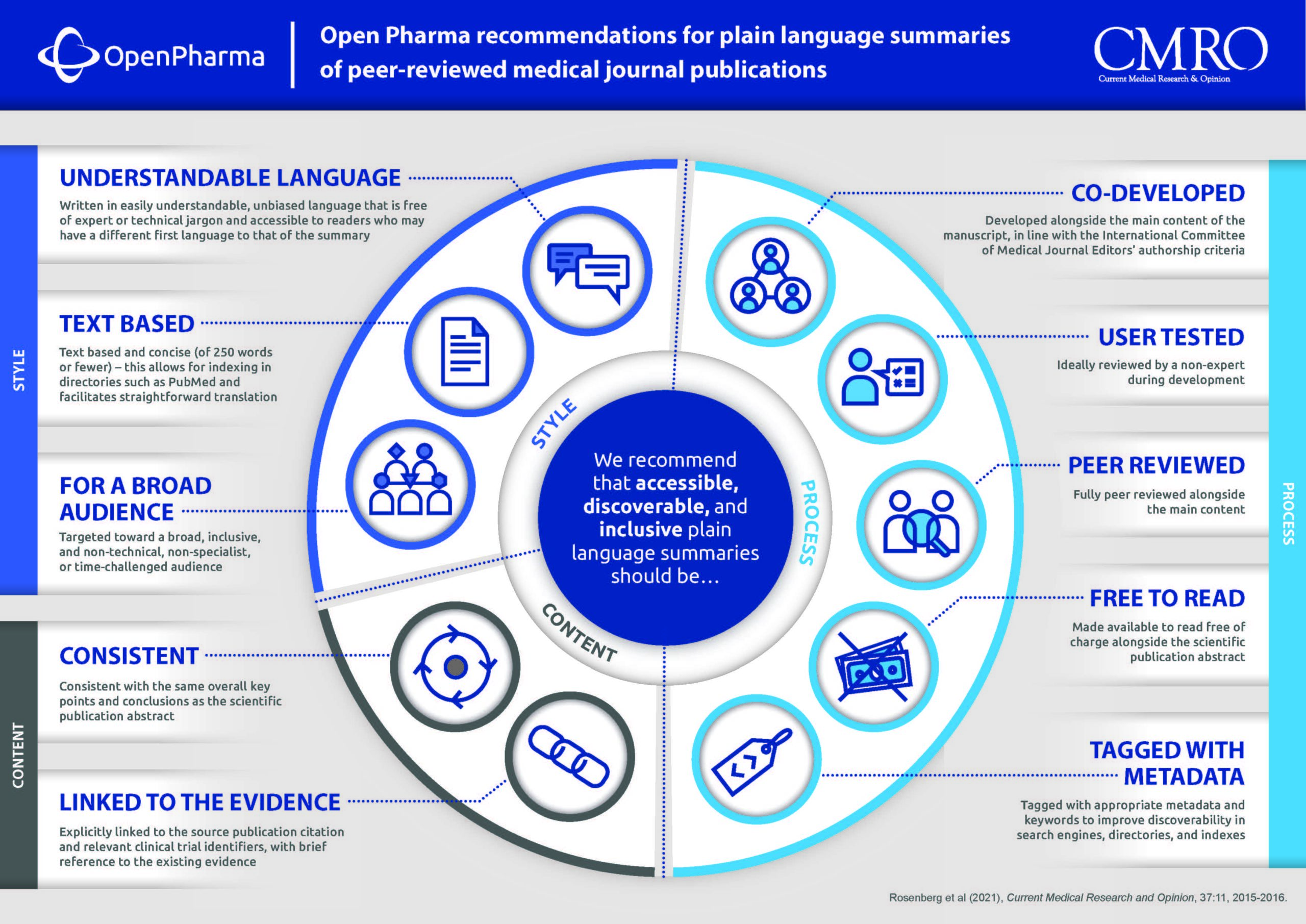
As a minimum standard, we recommend that PLS are:
- Targeted toward a broad, inclusive, and nontechnical, nonspecialist, or time-challenged audience
- Written in easily understandable, unbiased language that is free of expert or technical jargon and accessible to readers who may have a different first language to that of the summary
- Text based and concise (of 250 words or fewer)—this allows for indexing in directories such as PubMed and facilitates straightforward translation
- Explicitly linked to the source publication citation and relevant clinical trial identifiers, with brief reference to the existing evidence
- Consistent with the same overall key points and conclusions as the scientific publication abstract
- Developed alongside the main content of the manuscript, in line with the International Committee of Medical Journal Editors’ authorship criteria
- Ideally reviewed by a nonexpert during development
- Fully peer reviewed alongside the main content
- Made available to read free of charge alongside the scientific publication abstract
- Tagged with appropriate metadata and keywords to improve discoverability in search engines, directories, and indexes
Since the publication of the recommendations, Open Pharma continues to perform research and provide thought leadership on various aspects of PLS, most recently on indexing practices and journal policies and attitudes.
What Could Be Next for Editors and Publishers?
We hope that the Open Pharma recommendations will encourage editors and publishers to include PLS in more biomedical publications, with at least a text-based PLS included as the norm. We strongly believe all journals—but particularly biomedical journals—should be offering PLS options for all manuscripts and ensuring that they are correctly indexed on PubMed. Journals could even require PLS in the same way as technical abstracts are a given requirement. While many journals may be willing to accept PLS if directly queried, these policies need to be explicitly provided in author guidelines, including formatting details such as word count, to guide drafting.
We acknowledge that there are very reasonable hesitations and practical barriers to rolling out PLS offerings. We therefore encourage those with questions, concerns, comments. and ideas to join in the conversation and contribute to the evolving practice. Reach out and get involved with existing PLS initiatives, use the hashtag #PlainLanguageSummaries on Twitter and LinkedIn, talk to colleagues with PLS experience to get their perspectives, and talk to colleagues without PLS experience to bring them into the discussion. Join in.
Acknowledgments
The author thanks Tim Koder of Oxford PharmaGenesis and Members of the Open Pharma PLS Working Group for reviewing a version of this article: Slávka Barónikova of Galápagos, Valérie Philippon of Takeda, and Allison Spinelli of Janssen. The author also thanks Velissaria Vanna of Oxford PharmaGenesis for providing editorial support.
References and Links
- FitzGibbon H, King K, Piano C, Wilk C, Gaskarth M. Where are biomedical research plain-language summaries? Health Sci Rep. 2020;3(3):e175. https://doi.org/10.1002/hsr2.175.
- Gattrell W, Wager K, Sheikh N, Chisholm A. Prevalence and characteristics of plain language summaries indexed in PubMed. Original abstracts from the 2022 European Meeting of ISMPP. Curr Med Res Opin. 2022;38(sup1):25–45. https://doi.org/10.1080/03007995.2022.2044117.
- Besançon L, Peiffer-Smadja N, Segalas C, Jiang H, Masuzzo P, Smout C, Billy E, Deforet M, Leyrat C. Open science saves lives: lessons from the COVID-19 pandemic. BMC Med Res Methodol. 2021;21:117. https://doi.org/10.1186/s12874-021-01304-y.
- 3M. State of science index survey. [accessed March 29, 2022]. https://www.3m.com/3M/en_US/state-of-science-index-survey/interactive-3m-state-of-science-survey/.
- United Nations. Universal declaration of human rights. 1948. Article 27(1).
- Ipsos Mori. Wellcome Trust monitor report, wave 3. [accessed March 29, 2022]. https://doi.org/10.6084/m9.figshare.3145744.
- Selden CR, Zorn M, Ratzan S, Parker RM. Health literacy. Curr Bibliogr Med. 2000;1. [accessed March 29, 2022]. https://www.nlm.nih.gov/archive/20061214/pubs/cbm/hliteracy.pdf.
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National action plan to improve health literacy. [accessed March 29, 2022]. https://health.gov/sites/default/files/2019-09/Health_Literacy_Action_Plan.pdf.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The health literacy of America’s adults: results from the 2003 National Assessment of Adult Literacy. US Department of Education, National Center for Education Statistics. 2006;483 [accessed 2022 March 29]. https://nces.ed.gov/pubs2006/2006483.pdf.
- Rowlands G, Protheroe J, Winkley J, Richardson M, Seed PT, Rudd R. A mismatch between population health literacy and the complexity of health information: an observational study. Br J Gen Pract. 2015;65(635):e379–e386. https://doi.org/10.3399/bjgp15X685285.
- Sørensen K, Pelikan JM, Röthlin F, Ganahl K, Slonska Z, Doyle G, Fullam J, Kondilis B, Agrafiotis D, Uiters E, et al. Health literacy in Europe: comparative results of the European health literacy survey (HLS-EU). Eur J Publ Health. 205;25(6):1053–1058. https://doi.org/10.1093/eurpub/ckv043.
- National Voices. The report from the People and Communities Board to the Chief Executive of NHS England. [accessed March 29, 2022]. https://www.nationalvoices.org.uk/sites/default/files/public/publications/a_new_relationship_with_people_and_communities_0.pdf.
- Lobban D, Oliver J, Buttaro M, Falleni D, McGrath M. Do healthcare professionals really value plain language summaries? Original abstracts from the 2022 European Meeting of ISMPP. Curr Med Res Opin. 2022;38(sup1):25–45. https://doi.org/10.1080/03007995.2022.2044117.
- Procter J, Galbraith R, Moloney C. Talking the patients’ language: the importance of effective, health literate, patient-centred engagement. IQVIA White Paper. [accessed April 27, 2022]. https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/talking-the-patients-language.pdf
- Pushparajah DS, Manning E, Michels E, Arnaudeau-Bégard, C. Value of developing plain language summaries of scientific and clinical articles: a survey of patients and physicians. Ther Innov Regul Sci. 2018;52(4):474–481.https://doi.org/10.1177/2168479017738723.
- Maurer M, Siegel JE, Firminger KB, Lowers J, Dutta T, Chang JS. Lessons learned from developing plain language summaries of research studies. Health Lit Res Pract. 2021;5(2):e155–e161. https://doi.org/10.3928/24748307-20210524-01.
- Edgell C, Rosenberg A. Putting plain language summaries into perspective. Curr Med Res Opin. 2022;38:871–874. https://doi.org/10.1080/03007995.2022.2058812.
- Winter S, Halford C, West M, Evans M. Do plain language summaries encourage readers to access your publication? A pilot study. Original abstracts from the 2022 European Meeting of ISMPP. Curr Med Res Opin. 2022;38(sup1):25–45. https://doi.org/10.1080/03007995.2022.2044117.
- Turner L, Shamseer L, Altman DG, Schulz KF, Moher D. Does the use of the CONSORT Statement impact the completeness of reporting od randomised controlled trials published in medical journals? A Cochrane review. Syst Rev. 2012;1:60. https://doi.org/10.1186/2046-4053-1-60.
- Lobban D, Gardner J, Matheis R. Plain language summaries of publications of company-sponsored medical research: what key questions do we need to address? Curr Med Res Opin. 2021;38(2)189–200. https://doi.org/10.1080/03007995.2021.1997221.
- Silvagnoli LM, Shepherd C, Pritchett J, Gardner J. Optimizing readability and format of plain language summaries for medical research articles: cross-sectional survey study. J Med Internet Res. 2022;24(1):e22122. https://doi.org/10.2196/22122.
- Khobragade S, Perrett P, Burgess C, Smith H, Eade D, Cheshire A, Law L. Analysis of reach for different formats of plain language summaries. Original abstracts from the 2022
European Meeting of ISMPP. Curr Med Res Opin. 2022;38(sup1):25–45. https://doi.org/10.1080/03007995.2022.2044117. - Foulcer S, Sheppard D, Gokool N. Seek and you will [potentially] find: the impact of plain summary accessibility on engagement and wider dissemination. Original abstracts from the 2022
European Meeting of ISMPP. Curr Med Res Opin. 2022;38(sup1):25–45. https://doi.org/10.1080/03007995.2022.2044117. - Patient Focused Medicines Development. Plain language summaries (PLS) of peer-reviewed publications and conference presentations: practical ‘How-To’ guide for multi-stakeholder co-creation. [accessed March 30, 2022]. http://pemsuite.org/How-to-Guides/WG5.pdf.
- Envision Pharma Group. Plain language summaries (PLS) of publications toolkit. [accessed March 30, 2022]. https://www.envisionthepatient.com/plstoolkit.
- Adis. Guidelines for digital features and plain language summaries. [accessed March 30, 2022]. https://springerhealthcare.com/wp-content/uploads/2022/01/Guidelines-for-digital-features-and-plain-language-summaries-v1.3.pdf.
- Dove Press. Manuscript structure. [accessed March 30, 2022]. https://www.dovepress.com/author-guidelines/manuscript-structure.
- Future Science Group. Future Medicine author guidelines. [accessed March 30, 2022]. https://www.futuremedicine.com/pb-assets/Future-Medicine-Author-Guidelines-1626446908487.pdf.
- Taylor & Francis Group. How to write a plain language summary. [accessed March 30, 2022]. https://authorservices.taylorandfrancis.com/publishing-your-research/writing-your-paper/how-to-write-a-plain-language-summary/.
- Rosenberg A, Baróniková S, Feighery L, Gattrell W, Olsen RE, Watson A, Koder T, Winchester C. Open Pharma recommendations for plain language summaries of peer-reviewed medical journal publications. Curr Med Res Opin. 2021;37(11);2015–2016. https://doi.org/10.1080/03007995.2021.1971185.
- Rosenberg A, Baróniková S, Feighery L, Gattrell W, Olsen RE, Watson A, Koder T, Winchester C. Infographic: Open Pharma recommendations for plain language summaries of peer-reviewed medical journal publications. Curr Med Res Opin. 2022;38:881–882. https://doi.org/10.1080/03007995.2022.2072570.
Adeline Rosenberg (ORCID: 0000-0003-4599-4291) is a Senior Medical Writer at Oxford PharmaGenesis Ltd, Oxford, UK, which facilitates Open Pharma.
Opinions expressed are those of the authors and do not necessarily reflect the opinions or policies of the Council of Science Editors or the Editorial Board of Science Editor.
